How To Calculate Vapor Pressure Using Enthalpy Of Vaporization
Predicting the Enthalpy of Vaporization and Calculating the Entropy of Vaporization of 87 Octane Gasoline Using Vapor Pressure level ()
Abstract
The vapor pressure (VP) of 87 course gasoline was measured using an enhanced VP acquisition system over a temperature range of approximately 19.0℃ (292.2 K) and 69.0℃ (342.2 K). The empirical data were used to predict the thermodynamic entities the enthalpy of vaporization ( ΔH vap ) and the entropy of vaporization ( ΔS vap ) of gasoline. The results of this investigation yielded a ΔH vap value of 35.1 kJ/mol and ΔS vap of 102.5 J/mol·K. The value of ΔH vap was in excellent agreement with the findings of a prior study (Balabin et al ., 2007), which produced a ΔH vap values of 37.3 kJ/mol and 35.4 kJ/mol. The enthalpy and entropy of vaporization of n-heptane (37.2 kJ/mol and 100.1 J/mol·M) and n-octane (39.1 kJ/mol and 98.iii J/mol·One thousand) were besides determined after acquiring VP data for the two VOCs. The empirical results for north-heptane and north-octane were too in excellent agreement with the literature. These favorable comparisons strengthen the chapters of our system for acquiring the VP data for pure and volatile multi-component mixtures.
Share and Cite:
Abernathy, S. and Dark-brown, Chiliad. (2016) Predicting the Enthalpy of Vaporization and Calculating the Entropy of Vaporization of 87 Octane Gasoline Using Vapor Pressure. Open Access Library Journal, 3, one-eleven. doi: 10.4236/oalib.1102954.

1. Introduction
Automotive gasoline is a major production manufactured by the petroleum industry. The vapor pressure (VP) is a key physical property of automotive gasoline likewise equally aviation fuels. Gasoline is a petroleum fuel that is highly volatile. It is refined product of rough oil consisting of a mixture of hydrocarbons, additives, and blending agents. The VP is critically important for both automotive and aviation gasoline; this is due to these fuels being manufactured as liquids, but consumed in the vapor stage. Thus, high volatility is a prerequisite for a gasoline to ensure sufficient engine start-upwards, warm-up and dispatch under routine driving and flying conditions [1] [ii] . The VP of gasoline is likewise vital for predicting the concentration of volatile compounds discharged into the temper via the combustion of the fuel.
In that location are a number of methods for determining the VP and they are somewhat time- consuming. The Reid vapor pressure (RVP) is typically the measurement of choice for ascertaining the volatility of commercial gasoline in the refinery industry. The protocol and apparatus for acquiring the RVP are well described in ASTM D-323. The RVP is the absolute vapor pressure exerted by a mixture as determined at a temperature of 100˚F (37.8˚C/311.0K) and a vapor to liquid ratio of 4:1. It differs from the true vapor pressure (TVP) which is described as the pressure exerted by a vapor in equilibrium with its liquid phase at a specific temperature. Measuring the TVP is probably more conducive for determining the concentration of combustion contaminants emitted into the atmosphere since a maximum threshold has been imposed for the VP of gasoline in club to restrict air pollution [1] - [three] .
The petroleum industry relies heavily on performing simulations in order to test and optimize processes prior to the manufacturing of gasoline [4] . This is done in lodge to ensure the gasoline to meet technological specifications and stringent environmental regulations mandated to improve air quality. High quality empirical VP data, as a office of temperature, and thermodynamic information are crucial input parameters for the optimization of these models. The enthalpy of vaporization (∆Hvap) is i of these vital thermodynamic parameters since information technology is one of several characteristics that describe the volatility of a fuel. Predicting the corporeality of heat required to evaporate (i.e. vaporizes) a specific quantity of gasoline to produce an ignitable air-fuel-vapor mixture is of the utmost importance for automobile engines; this is particular important to know before conception of a gasoline blend and it existence released to market place [4] . Equally important is the utilize of analytical equations to produce input parameters for models. These equations also serve to compute stand up-alone thermodynamic information of gasoline blends. An fantabulous outline of numerous belittling expression used in this capacity was summarized by Riazi et al. [3] .
At that place is a large database of enthalpy of vaporization (∆Hvap) values for pure volatile organic compounds (VOCs). This data can exist readily plant in reference books such as the CRC (Chemical Safe Company) Handbook of Chemistry and Physics and Perry & Green Chemical Engineers Handbook. However, there is a lack of ∆Hvap information on circuitous mixtures such as gasoline and aviation fuel; this may be due to before studies not being well adult every bit suggested by Chapka et al. [5] . Withal, there is a plethora of Reid and true vapor pressure level data on numerous blends of gasoline [6] . The Clausius-Clapeyron equation (i.due east. plot of lnP vs. 1/T(G)) could exist readily applied to this gasoline VP information in lodge to create preliminary ∆Hvap values besides as construct a database. This precursory information would facilitate filling this particular knowledge gap.
2. Experimental
2.1. Materials
The gasoline sample used in this work was purchased at a local Sunoco gas station, and had an octane rating of 87. The composition and information on the ingredients of the gasoline are listed on its cloth condom data canvass. The VOCs northward-heptane (99% HPLC grade), northward-octane, anhydrous ethanol (EtOH), toluene were purchased from the Aldrich Chemical Visitor and Fischer Scientific. These materials, gasoline and chemicals, were used without purification and handled using proper safety measures as specified from their Material Safe Data Sheet.
2.2. Methodology
The VP data of gasoline along with n-heptane (99% HPLC grade), northward-octane, distilled h2o, EtOH, and toluene was measured utilizing an enhanced VP acquisition system, which is an innovative modification of the "Humid-Bespeak Method" apparatus. In this process, liquid vapors are in equilibrium with its boiling liquid at a specific externally applied pressure. The method is well recognized in the literature [eight] . A portable Welch GEM direct-bulldoze vacuum pump was used as the external vacuum source to vary the pressure of the conquering organisation. A digital vacuum regulator (DVR) manufactured by J-KEM Scientific, connected to the vacuum pump, was utilized to accurately control the pressure level (±0.5 torr) higher up the gasoline sample (100 ml) in the liquid reservoir (250 ml circular-bottom flask).
The heating of the gasoline sample was carried out using a Büchi model B-490 h2o bath interfaced with a J-KEM Scientific digital temperature controller (DTC). The liquid reservoir was submerged in the water bath to equilibrate the sample at a predetermined fix temperature in which the VP would be measured. The temperature of the sample was measured by the thermocouple sensor component (±0.five˚C) of the DTC, which was positioned in the middle of the sample. The DVR and DTC were interfaced to a desktop PC, which logged into an excel spreadsheet the temperature and pressure data in real-time every 20 seconds. The resulting VP data can be readily evaluated in the excel spreadsheet or exported for processing using the KaleidaGraph software package. A minimum of nine VP measurements were acquired at each specified temperature to ensure reproducibility of the data; this would stand for to a total minimum acquisition time of three minutes. There were between nine and 30 VP measurements acquired at each temperature interval throughout this investigation. The standard divergence in force per unit area (torr) and temperature (C) is shown in Table 1 for h2o and the each VOC. These units of temperature and pressure were chosen to be consisted with the literature in which they were compared. A detailed illustration of the enhanced VP acquisition arrangement was depicted in a prior publication in this journal [7] .
3. Result and Give-and-take
In this investigation, the enhanced VP acquisition system was used to collect empirical VP data of Sunoco 87 form gasoline, n-heptanes, and northward-octane. Calibration of the system using distilled water, EtOH, and toluene have too been reported. The information also served every bit a reference indicator, and is displayed with all the results of this investigation in Table one. It can exist readily observed that the experimental data is inline with the literature information [9] , which are shown in parentheses in Tabular array 1.
Determining the vapor force per unit area of a pure liquid or mixture is rooted in agreement the existence of a liquid-vapor boundary. A dynamic equilibrium exists betwixt the two phases, i.e. the liquid and vapor phases; this is an interface in which the pressures and temperatures of the 2 phases can co-exist. The Clapeyron equation is an exact expression for the slope of the liquid-vapor phase boundary. Equation (ane) represents the equilibrium that occurs between the liquid-vapor phases, where the gradient of the purlieus is dP/dT:
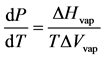 (1)
(1)
In this equation, ΔHvap is the modify in the enthalpy of vaporization and T is the absolute the temperature in Kelvin. The variable ΔVvap stand for to the change in molar book of vaporization, where nosotros assume the gas phase behaves equally an platonic gas. If we infer that ΔVvap ≈ 5vap and use the ideal equation of state, we tin derive Equation (2):
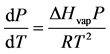 (2)
(2)
The variable P in Equation (2) is the vapor pressure and R is the gas constant (8.314 J/mol∙Chiliad). Past separating the variables P and T, integration of Equation (two) yields the Clausius-Clapeyron equation; this well known phase equilibrium expression is repre- sented by Equation (3)
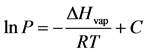 (3)
(3)

Table 1. Summary of predicted ΔHVap and computed ΔSVap results from VP information amassed from the Enhanced VP Acquisition Organization.
The enthalpy of vaporization tin can be predicted from Equation (three) from a plot of the natural logarithm of pressure (lnP) versus the reciprocal accented temperature (1/Yard). A straight line is generated with a gradient equal to −ΔHvap/R and an intercept denoted by the constant C [8] . This is the methodology employed in this study to predict ∆Hvap Equally, the value of ∆Svap for each liquid was computed using Trouton'due south Constabulary and is expressed by Equation (4):
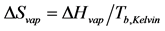 (4)
(4)
Figure 1 illustrate plots of the empirical VP data of the calibration and reference liquids water, EtOH, and toluene as the lnP vs. 1/T(Yard). A linear least square regression fit was applied to each gear up of VP data and is depicted in Effigy 1. Using the water VP data equally an instance, a linear least square regression fit of the information results in an equation for the line of all-time fit equal to y = 20.657 − 5215.1x. This can be translated to the form of Equation (five) as:
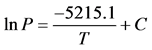 (5)
(5)
The enthalpy of vaporization (∆Hvap) and entropy of vaporization (∆Svap) of the reference liquids were determined prior to that of the 87 grade gasoline. This was completed in order to larn the optimal VP information as well equally ensure maximum operation of the VP acquisition system. Using the gradient of the linear square regression fit of the water VP data, a value of 43.4 kJ∙mol−one was evaluated as the enthalpy of vaporization (∆Hvap) of water; this deviated past approximately 1.0% from the literature value [nine] . The entropy of vaporization was determined using Equation (four) (Trouton'due south Law) past dividing ∆Hvap of water by its normal humid bespeak temperature in Kelvin (100.0˚C, 373.two 1000); this resulted in a ∆Svap of 116.3 J/mol∙1000 for water. The predicted values of ∆Hvap for EtOH and toluene were 42.0 kJ/mol and 35.3 kJ/mol respectively using the line of best fit through the empirical VP information. The ∆Hvap of EtOH and toluene deviated by 1.0 and 5.iv% respectively, and were in excellent understanding with the literature [9] . The computed values for ∆Svap of EtOH and toluene were 119.5 J/mol∙K and 35.3 J/mol∙Yard respectively. Although this data is from a previously published study by the authors, the inclusion of information technology in this paper strengthens and validates the performance as well as the use of the enhanced VP acquisition system.
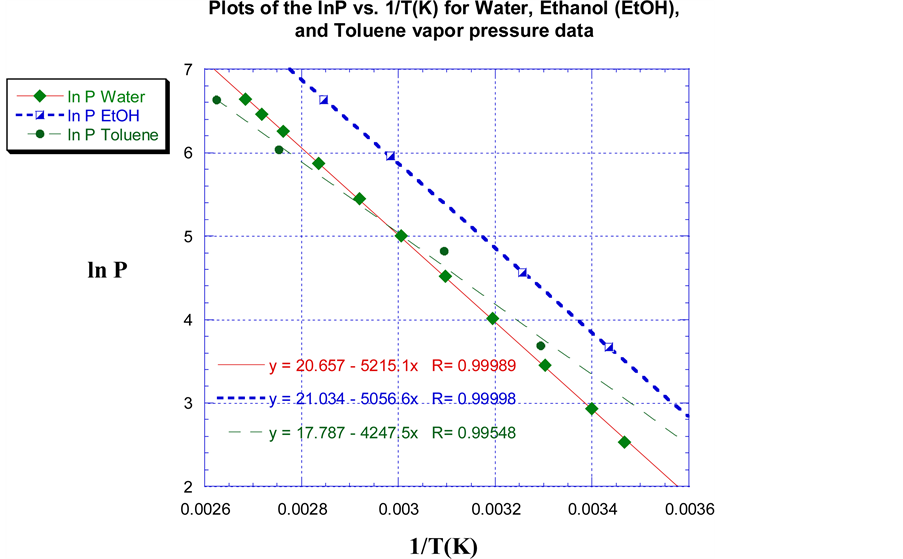
Figure 1. Plots of the lnP vs. 1/T(K) of Water, Ethanol, and Toluene and their linear least squares regression fit of the empirical VP information. Water, EtOH, and Toluene data are represented by light-green diamonds, half-filled (white/blue) squares, and light-green circles respectively.

Figure 2. A plot of the lnP vs. 1/T(K) of 87 octane rating Sunoco gasoline and the linear to the lowest degree squares regression fit of the empirical VP data.
amassing VP information.
VP data was also amassed for n-heptane and n-octane in this report. Heptane was selected since it is used along with iso-octane to modify the octane rating of gasoline blends. In 1927, the octane rating was developed in society to control spontaneous combust of gasoline blends that produce a knocking audio in a standard engine. The direct northward-heptanes caused severe knocking in an engine and assigned a rating of 0. In contrast, iso-octane (2,2,four-trimethylpentane) was assigned an octane rating of 100 since it did not cause knocking in engines [eleven] . During this investigation, VP measurements were acquired for n-octane (octane rating of −xx) due to the lack of iso-octane.
The values for ∆Hvap and ∆Svap for northward-heptane and n-octane were predicted and computed in the same style as the Sunoco 87 grade gasoline. An 87 octane gasoline can be described every bit having the knock resistance as a mixture of xiii% n-heptane and 87% iso-octane by volume (v/v) respectively. In Figure iii, the linear to the lowest degree square regression fit of north-heptane yielded a slope of −4476.6 with a correlation coefficient (R) of 0.9994. A value for ∆Hvap of 37.2 kJ/mol was predicted, and a ∆Svap of 100.1 J/mol∙K was computed using Trouton'south Law. These values were within iii.v% of the literature values [12] . For n-octane, a least foursquare fit of the VP data every bit shown in Figure iv resulted in a slope of −4698.8 and a correlation coefficient of 0.99935. A value of 39.1 kJ/mol for ∆Hvap was predicted, and a ∆Svap value of 98.iii J/mol∙K resulted from this information. Both of these values were inside 1.0% of their literature value [xiii] .
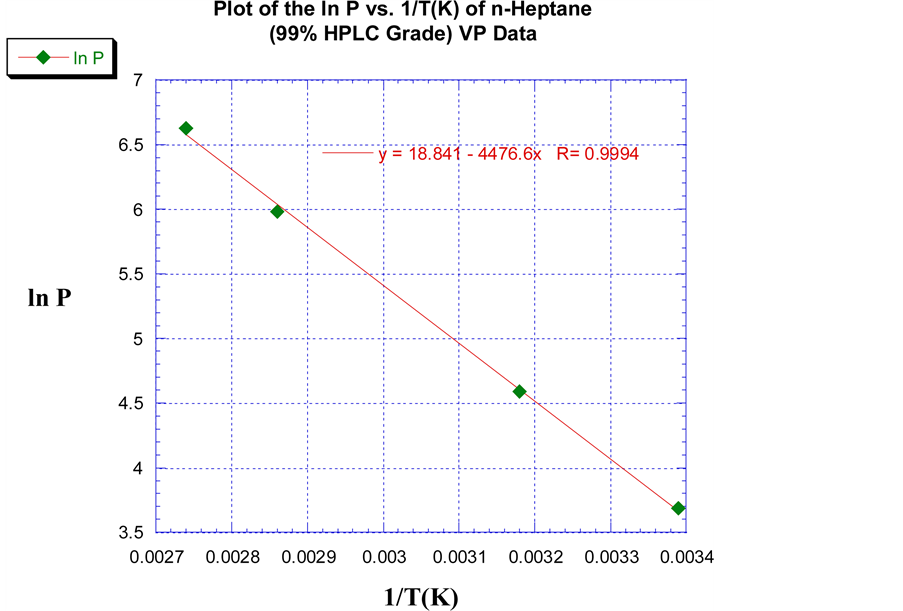
Figure 3. A plot of the lnP vs. 1/T(K) of northward-Heptane and the linear least squares regression fit of the empirical VP data.

Figure 4. Plots of the lnP vs. 1/T(1000) of northward-octane and iso-octane (2,ii,4-trimethylpentane) and their linear least squares regression. The north-octane plot is of experimental VP data while the iso-octane is literature information. The due north-octane is represented by half-filled (white/blue) squares and iso-octane by green diamonds.
A linear square regression fit was also applied to literature VP data of iso-octane [vii] . This plot is shown in Figure four along with n-octane linear square regression fit of its empirical VP information. By examining the plots, information technology is readily apparent that the experimental and literature data practise not overlap. However, the two lines are parallel all the same their slopes are different; this indicates that ii distinct values of ΔHvap and ∆Svap for n-octane and iso-octane (2,2,4-trimethylpentane) will be predicted and calculated. An enthalpy of vaporization (ΔHvap) value of 36.4 kJ/mol was predicted from the linear least squares fit of the iso-octane literature data. The ∆Svap for iso-octane 97.eight J/mol∙Grand was computed from the literature. These values were within 3.5% when compared to the study by Wu et al. [14] [15] .
Table ane is a summation of the VP data clustered for water, EtOH, n-heptane, n- octane, toluene, and Sunoco 87 form gasoline. Information technology contains the predicted value of ∆Hvap, the computed values of ∆Southwardvap, the slope of the line fit, and the correlation coefficient. Information technology is important to notice that the correlation coefficient R for all of the linear to the lowest degree square regression fits are shut to the value ane. From Table 1, it is readily apparent that the experimental data parallels the literature information, which are shown in parentheses. The results of this investigation clearly demonstrate the exceptional adequacy and efficiency of our enhanced VP acquisition system for amassing VP information. We believe that this considerable trunk of original VP data would be invaluable to the petroleum industry since simulations of ΔHvap are utilized extensively for evaluating and the optimization of processes in the manufacturing of gasoline blend [four] [16] .
4. Conclusion
Vapor pressure data were obtained for 87 grade gasoline using the enhanced VP conquering system described in this work. VP measurements were also acquired for n-hep- tane and n-octane, which served every bit reference and system calibration data. The VP data were also used to predict the enthalpy (∆Hvap) and entropy of vaporization (∆Due southvap) of these VOCs from their line fits using the Clausius-Clayperon equation and Troutons Police force respectively. The VP information and the predicted also every bit calculated thermodynamic results were in splendid agreement with the literature results. This work has reinforced the validity and practicality of the enhanced VP conquering organisation as an efficient tool.
Acknowledgements
The authors admit Howard University, Dr. Clarence Lee (Executive Director) of the Howard University LS-AMP (Louis Stokes Alliance for Minority Participation) Program, Marquia Whitlock (LS-AMP Plan), Monique Yvette McClung (LS-AMP Program), NSF (Grant Number HRD-1000286), and NIH-NIGMS (Grant Number T34GM105660).
Conflicts of Interest
The authors declare no conflicts of interest.
References
| [i] | ASTM (1999) Annual Book of Standards. American Social club for Testing and Materials, Philadelphia. |
| [ii] | da Silva, R., Cataluna, R., de Menezes, East.West., Samios, D. and Piatnicki, C.M.Southward. (2005) Effect of Additives on the Antiknock Properties and Reid Vapor Force per unit area of Gasoline. Fuel, 84, 951-959. http://dx.doi.org/10.1016/j.fuel.2005.01.008 |
| [3] | Riazi, M.R., Albahri, T.A. and AlQattan, A.H. (2003) Prediction of the Reid Vapor Pressure of Petroleum Fuels. Fuel Chemistry Division Preprints, 48, 478-479. |
| [4] | Farsibaf, G.M., Golchinpour, M. and Barzegar, A. (2011) Global Optimization in Order to Notice Blend Composition of Gasoline of Desired Octane Number Considering Ethanol as Octane-Booster. Proceeding of the 41st International Conference on Computers & Industrial Technology. |
| [5] | Chupka, G.M., Christensen, E., Fouts, L., Alleman, M.A., Matthew, A.R. and McCormick, R.L. (2015) Rut of Vaporization Measurements for Ethanol Blends upwardly to 50 Book Percent in Several Hydrocarbon Blendstocks and Implications for Knock in SI Engines. SAE International Journal of Fuels and Lubricants, 8, 251-263. http://dx.doi.org/10.4271/2015-01-0763 |
| [6] | Andersen, Five.F., Andersen, J.Due east., Wallington, T.J., Mueller, S.A. and Nielsen, O.J. (2010) Vapor Pressure of Alcohol-Gasoline Blends. Energy Fuels, 24, 3647-3654. http://dx.doi.org/10.1021/ef100254w |
| [7] | Abernathy, Due south.1000. and Brown, G.R. (2015) Using the Vapor Pressure of Pure Volatile Organic Compounds to Predict the Enthalpy of Vaporization and Calculating the Entropy of Vaporization. Open Access Library Periodical, 2, e1927. http://dx.doi.org/ten.4236/oalib.1101927 |
| [eight] | Garland, C.Westward., Nibler, J.Westward. and Shoemaker, D.P. (2009) Experiments in Physical Chemistry. eighth Edition, McGraw-Loma, New York. |
| [9] | Weast, R.C., Astle, M.J. and Beyer, W.H. (1984) CRC Handbook of Chemistry and Physics. In: Eds., R.C., CRC Press, Boca Raton, D199-D214. |
| [10] | Balabin, R.G., Syunyaev, R.Z. and Kapov, S.A. (2007) Tooth Enthalpy of Vaporization of Ethanol-Gasoline Mixtures and Their Colloid State. Fuel, 86, 323-327. http://dx.doi.org/x.1016/j.fuel.2006.08.008 |
| [11] | Girard, J.E. (2005) Principles of Environmental Chemical science. Jones and Bartlett Publishers, MA. |
| [12] | Sipowska, J.T. and Wieczorek, S.A. (1984) Vapour Pressure level and Excess Gibbs Free Energies of (Cyclohexanol + northward-Heptane) between 303.147 and 373.278 Thousand. Journal of Chemic Thermodynamics, xvi, 693-699. http://dx.doi.org/10.1016/0021-9614(84)90051-X |
| [13] | Ewing, M.B. and Ochoa, J.C. (2003) The Vapour Force per unit area of n-Octane Deter-mined Using Comparative Ebulliometry. Fluid Phase Equilibria, 210, 277-285. http://dx.doi.org/10.1016/S0378-3812(03)00174-2 |
| [14] | Wu, H.S., Pividal, Yard.A. and Sandler, S.I. (1991) Vapor-Liquid Equilibria of Hydrocarbons and Fuel Oxygenates. Periodical of Chemical & Engineering Information, 36, 418-421. http://dx.doi.org/ten.1021/je00004a021 |
| [fifteen] | http://webbook.nist.gov/chemistry/formser.html |
| [sixteen] | Gopinathan, N. and Sarai, D.Northward. (2001) Predict Heat of Vaporization of Crudes and Pure Components Revised Ii. Fluid Stage Equilibria, 179, 277-284. http://dx.doi.org/10.1016/S0378-3812(00)00501-X |
How To Calculate Vapor Pressure Using Enthalpy Of Vaporization,
Source: https://www.scirp.org/journal/paperinformation.aspx?paperid=70527
Posted by: williamsyestan73.blogspot.com


0 Response to "How To Calculate Vapor Pressure Using Enthalpy Of Vaporization"
Post a Comment