Characteristics Of An Ionic Bond
four.7: Characteristics of Ionic Compounds
- Folio ID
- 221351
Learning Objectives
- To depict the physical properties of ionic compounds.
The figure beneath shows merely a few examples of the color and luminescence of naturally occurring ionic crystals. The regular and orderly organization of ions in the crystal lattice is responsible for the various shapes of these crystals, while transition element ions give ascent to the colors.

Physical Properties of Ionic Compounds
Melting Points
Because of the many simultaneous attractions betwixt cations and anions that occur, ionic crystal lattices are very strong. The process of melting an ionic compound requires the addition of large amounts of free energy in order to break all of the ionic bonds in the crystal. For example, sodium chloride has a melting temperature of nigh 800oC. As a comparison, the molecular compound water melts at 0 °C.
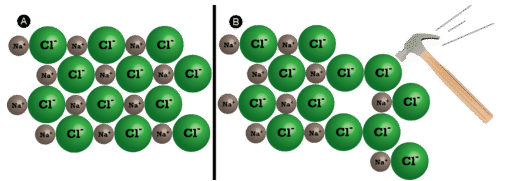
Shattering
Ionic compounds are generally difficult, merely brittle. Why? It takes a large amount of mechanical force, such as striking a crystal with a hammer, to forcefulness one layer of ions to shift relative to its neighbor. However, when that happens, it brings ions of the aforementioned charge next to each other (meet below). The repulsive forces between like-charged ions crusade the crystal to shatter. When an ionic crystal breaks, it tends to do and then along smooth planes because of the regular organization of the ions.
Solubility in water
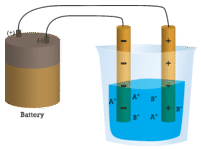
A considerable high number of ionic compounds are soluble in water. Table salt, or sodium chloride (NaCl), the most mutual ionic compound, is soluble in water (360 g/L). Remember that NaCl is a salt crystal equanimous not of detached NaCl molecules, but rather of an extended array of Na+ and Cl- ions spring together in 3 dimensions through electrostatic interactions. When a crystal of NaCl comes into contact with water, the water molecules collaborate with the Na+ and Cl- ions on the crystal'southward surface, equally shown in the figure \(\PageIndex{iii}\). As a consequence of the interaction betwixt the Na+ and Cl- ions and the water molecules, the electrostatic interactions within the crystal are broken.

Figure \(\PageIndex{iii}\): Dosilution of sodium chloride in water. Epitome by Ahazard.sciencewriter, CC BY-SA 4.0, via Wikimedia Commons
Conductivity
Another feature property of ionic compounds is their electrical conductivity . The figure below shows three experiments in which ii electrodes that are continued to a light bulb are placed in beakers containing 3 different substances.
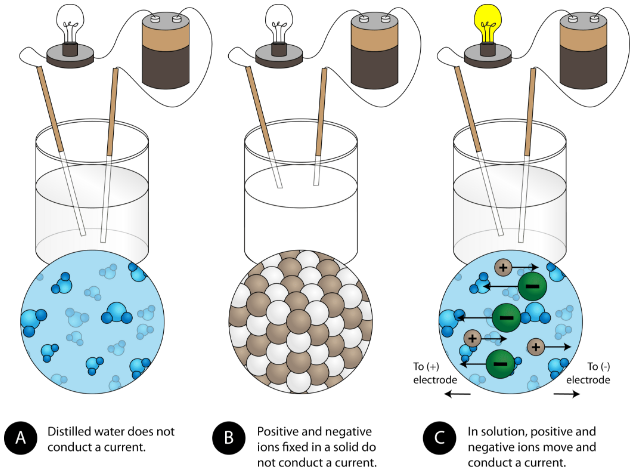
In the start chalice, distilled h2o does not conduct a current because water is a molecular chemical compound. In the second beaker, solid sodium chloride as well does non conduct a current. Despite beingness ionic and thus composed of charged particles, the solid crystal lattice does non let the ions to move betwixt the electrodes. Mobile charged particles are required for the circuit to be complete and the light seedling to calorie-free up. In the third beaker, the NaCl has been dissolved into the distilled h2o. At present the crystal lattice has been broken apart and the individual positive and negative ions tin motion. Cations motion to ane electrode, while anions move to the other, allowing electricity to flow (see figure below). Melting an ionic compound also frees the ions to behave a electric current. Ionic compounds conduct an electrical current when melted or dissolved in water.
Instance \(\PageIndex{i}\)
Write the dissociation equation of solid NaCl in water.
Solution
NaCl(s) → Na+(aq) + Cl–(aq)
Exercise \(\PageIndex{1}\)
Write the dissociation equation of solid NH4NO3 in water.
- Answer
-
NHivNO3(southward) → NHfour +(aq) + NO3 –(aq)
Key Takeaways
- Ionic compounds have loftier melting points.
- Ionic compounds are hard and brittle.
- Ionic compounds dissociate into ions when dissolved in water.
- Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials practise not.
- An ionic compound tin exist identified by its chemical formula: metal + nonmetal or polyatomic ions.
Contributors and Attributions
- CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Exercises
i. Explain how the bonding in an ionic solid explains some of the backdrop of these solids.
2. Which type(s) of solid conduct(southward) electricity in their liquid state only non in their solid state?
3. Based on chemical formula, identify which of the following is an ionic solid?
a. Hg
b. PH3
c. Ba(NO3)2
four. Based on chemical formula, identify which of the following will carry electric current in the melted land but not in the solid state?
a. HiiS
b. Si
c. KBr
5. Based on chemical formula, place which of the following will dissociate into ions when dissolved in water?
a. Co
b. CO
c. CaCl2
6. Write dissociation equations for the following ionic compounds.
- LiF(due south)
- CuSOfour(s)
- Na2Southward(southward)
- MgBr2(s)
Answers
1. The oppositely charged ions are very strongly held together, so ionic crystals take loftier melting points. Ionic crystals are as well breakable considering any baloney of the crystal moves aforementioned-charged ions closer to each other, so they repel. When melted and dissolved in solution, the ions become gratis to conduct electric current.
two. Ionic compounds
iii. Ba(NO3)2
4. KBr
v. CaClii
half dozen.
- LiF(s) → Li+(aq) + F–(aq)
- CuSO4(southward) → Cu2 +(aq) + SO4 2 –(aq)
- Na2S(s) → 2Na+(aq) + Southward2–(aq)
- MgBr2(s) → Mg2+(aq) + 2Br–(aq)
Characteristics Of An Ionic Bond,
Source: https://chem.libretexts.org/Courses/Brevard_College/CHE_103_Principles_of_Chemistry_I/04%3A_Chemical_Bond_I/4.07%3A__Characteristics_of_Ionic_Compounds
Posted by: williamsyestan73.blogspot.com


0 Response to "Characteristics Of An Ionic Bond"
Post a Comment